
Familiar with the pharmaceutical industry exhaust gas know, VOCs composition is not easy to recover, flow instability, production cycle. Therefore, in the process of treatment, the requirements will be very high, the following Australian Italian environmental protection to phenol, formaldehyde, triethylamine, aniline four kinds of typical VOCs exhaust gas related characteristics and exhaust gas treatment technology ideas to talk about.
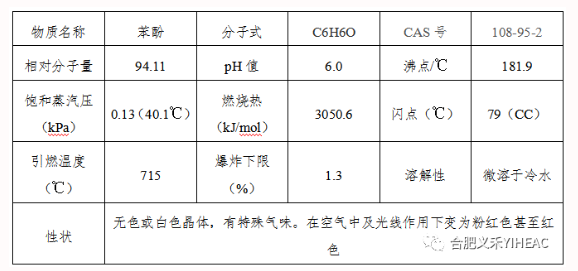
The weak acidity of phenol interacts with base to form salt. Most of its salts are water-soluble and can be dissociated by carbonic acid. This property can be used to distinguish phenols from carboxylic acids, which are used commercially to separate phenols from complex coal tar. Action of phenol with aqueous or alcoholic solution of ferric chloride. Blue or purplish blue. Liebermann chromogenic reaction occurs in most phenols when potassium nitrite is added to concentrated sulfuric acid. Phenol is prone to etherification or esterification, which can be used to produce phenyl acetate (C6H5OOCCH3), triphenyl phosphate [(C6H5)3PO4], phenyl salicylate (C6H5OOC6H5OH), anisole (C6H5OCH3), diphenyl ether (C6H5OC6H5), etc. Phenol is easily oxidized to form polyhydroxyl derivatives, biphenyl and diphenyl ether derivatives, quinones, oxalates and resinous substances.
Formaldehyde is the simplest aldehyde. It is usually classified as a saturated monaldehyde, but it is also equivalent to a binary aldehyde. In the reaction with the weak oxidant, each mole of HCHO can reduce up to 4mol of Ag or 2mol of cuprous oxide, which is twice the reducing capacity of acetaldehyde, so formaldehyde is like a binary aldehyde. The industrial product is usually 40% aqueous solution (containing 8% methanol), commonly known as formalin. Pure formaldehyde gas can be liquefied into liquid at -19℃. It can be miscible with non-polar solvents (e.g., toluene, ether, chloroform, ethyl acetate, etc.) in any proportion at low temperatures. The solubility decreases with increasing temperature. Formaldehyde can be burned and vapor and air can form an explosive mixture.
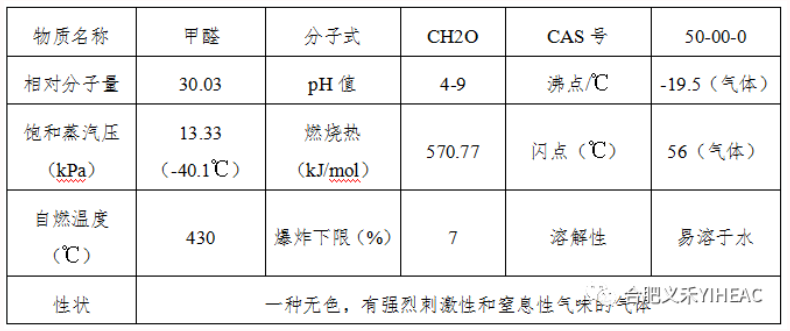
Pure formaldehyde has strong reducing effect, especially in alkali solution. Formaldehyde itself can be slow condensation reaction, especially prone to polymerization.

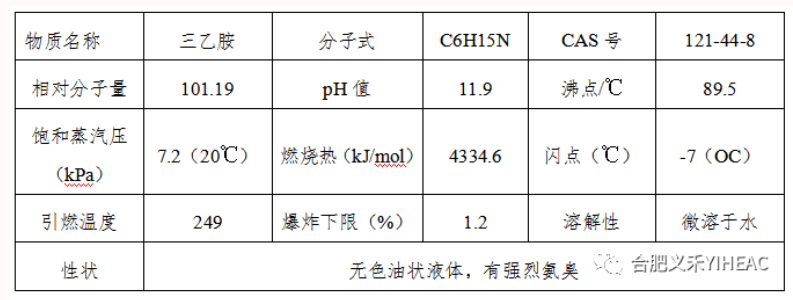
Triethylamine: highly toxic, having chemical properties similar to tertiary amine. The aqueous solution is basic and can react with halides to form quaternary ammonium salts. It is unstable to the oxidizer. The reaction with potassium permanganate is easy to oxidize and decompose, to produce acetic acid, ammonia and nitric acid. Oxidation with hydrogen peroxide produces triethylamine oxide. When pyrolysis at low pressure at 400℃, tetraethylhydrazine and butane are first produced, and then methane and nitrogen are produced. In the presence of cobalt, nickel, copper or copper chloride, alkyl exchange reaction with alcohol, to produce alkyl diethylamine, dialkyl ethyl amine, etc.
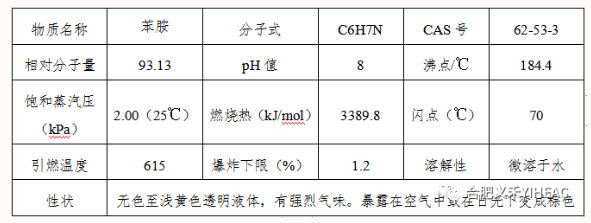
Compared with aliphatic amine, aniline is less basic, and the reaction with nitrous acid, amino group diazotization reaction to produce diazo salt. Diazo salts are conjugated to form azo compounds. Aniline neutralizes with inorganic acid to form water-soluble salt, and forms double salt with zinc chloride, copper, calcium chloride, etc. The alkylation reaction with alcohol in acidic solution gives the corresponding N-alkyl compound. The same reaction can occur with alkenes and alkanes halides. Aniline reacts with carboxylic acid, acid anhydride, acyl chloride, ester, etc., to form acylteaniline. Because of the presence of amino group in aniline, aromatic nuclei are prone to substitution reactions, such as alkylation, halogenation, sulfonation, nitrification, nitrosation and other reactions in ortho-or para-site. In addition, aniline is easy to oxidize, according to different conditions can produce p-benzoquinone, nitrobenzene, azobenzene, azobenzene oxide and so on. Aniline is hydrogenated to form cyclohexamine. React with aldehydes to form resinous condensates.
The pH value of the above four substances is greater than or less than 7, which makes the VOCs waste gas in the transport field should pay attention to anti-corrosion and electrostatic grounding, so the material selection of equipment needs to be comprehensively considered. In the treatment of these four substances, the characteristics of the substance itself can be used for classification treatment, such as formaldehyde is easy to dissolve in water, water absorption, aniline and phenol ignition temperature is high, high calorific value, RTO can be used for treatment, at the same time, oxidation can be used to generate heat, RTO self-sustaining combustion, reduce operating costs. Because triethylamine is highly toxic, we need to design RTO with negative pressure, which can prevent exhaust leakage. However, after oxidation of RTO, it should be noted that triethylamine and aniline are the precursor substances produced by fuel type nitrogen oxides. Therefore, alkaline washing can be designed at the back end of RTO, which can remove nitrogen oxides and reduce the exhaust gas temperature after purification. Protective end electrical equipment is damaged.
To sum up, this is a simple analysis of the four types of substances. In fact, in the actual production process, the waste gas generated is more complex. We can adapt to local conditions, detailed analysis and classified management, which can make the waste gas of the whole plant efficient, energy saving, safety and standardized management.
Online ConsultQQ

E-mail:
info@ssecn.com